Critical appraisal 2
This critical appraisal is about the article written by E. van Nood et al. in 2013. This study is an RCT and was performed to determine the advantage of donor-feces infusion compared with vancomycin treatment, both with and without bowel lavage. Since this article states an important improvement in therapy for recurrent Clostridium difficile infection (CDI) and the fact that we used this article as backbone in this weblog article, we want to critically evaluate it and check whether it was performed rightly.
Relevance research question
The
Research question of this article is: is donor-feces infusion compared with
vancomycin treatment, both with and without bowel lavage, a more effective
treatment for recurrent Clostridium Difficile infections. Feces donor infusion
has already been described as effective in many patients. However, there is a
lack of good randomized studies and therefore a gab in supporting data for this
treatment. To fill in those gabs randomized studies like these need to be done.
And thus is this a relevant research question.
Type of research question
This
research questions falls into the category of the 'questions about the
effectiveness of treatment'. In this case the effectiveness of the FMT compared
to vancomycin therapy, with or without bowel lavage. This states that the study
uses 1 intervention group: FMT with bowel lavage. And 2 control groups:
vancomycin with bowel lavage and vancomycin without bowel lavage. The study
population is mentioned: patients with recurrent CDI, but it is not mentioned
how many relapses the patients have. The clinical outcome is not mentioned in
the research question, but later in the article described as their primary
outcome: cure without recurrent CDI within 10 weeks after the start of therapy.
Study design
The study
was a not blinded randomized control trial in which 43 patients were randomly
assigned to received one of three therapies: 17 in the intervention group and
13 in both control groups. Study participants were recruited in a period of 2
years form the Amsterdam medical center (AMC) and were randomly assigned by the
study physicians. Patients needed to have a relapse of CDI and needed to be
treated for at least one course or antibiotic therapy. The CDI was defined by
diarrhea and a positive stool test for C dif. Toxin. Patients who were
immunocompromised, administered to the ICU or using antibiotics for other
infections than C. difficile were excluded from the study. All patients gave
written informed consent and the study was approved by the ethics committee of
the AMC. As mentioned before the study was not blinded, it may not be possible
to make a placebo for the FMT, since this is an fluid infusion method and
antibiotics need to be taken orally.
Bias
The
randomization was performed by the study physicians, but in the article
treatment allocation concealment was not mentioned. This could have influenced
the study results, since it is not sure if the study physicians knew which
patients could be addressed for which study group.
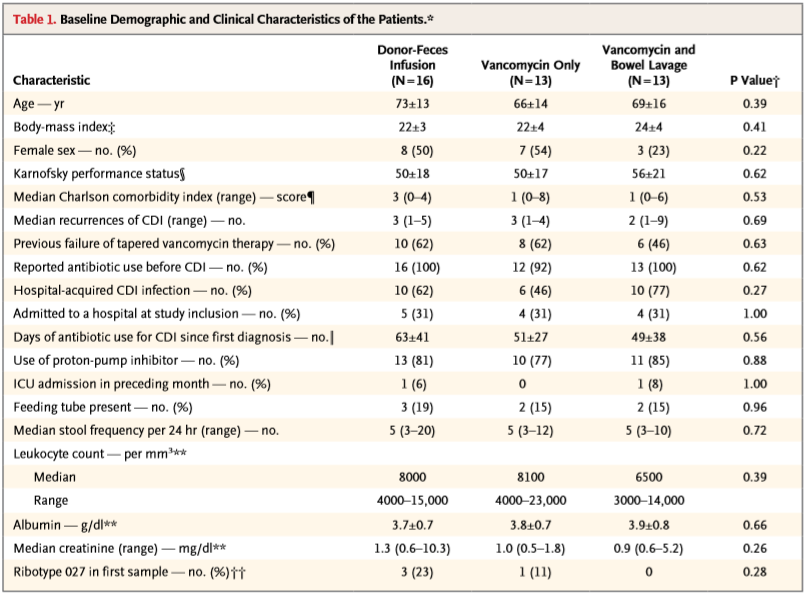
The
characteristics of the tree different study groups were not significantly
different as shown in table below.
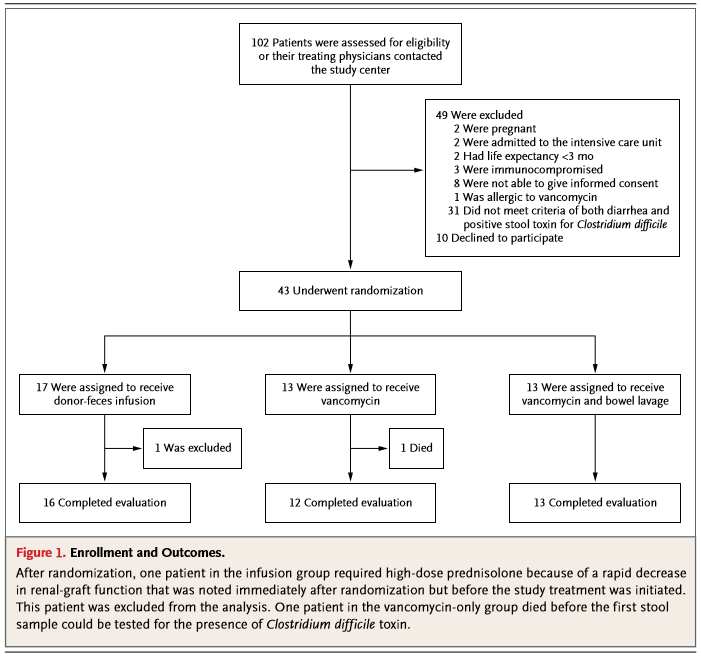
In the flow chart, you can see that 1 patient was excluded from the study after randomization. This particular patient required high-dose prednisolone because of a fast decrease in renal-graft function. Following the exclusion criteria the patient needed to be excluded. This happened before the treatment was initiated, so this had no influence on the study results.
In the
vancomycin without bowel lavage group one patient died before the first stool
sample could be tested. In the intention-to-treat analysis the vancomycin
therapy in this patients was considered a failure.
Original protocol?
The
original idea was to recruit 40 patients per study group. This number was
calculated by a power of 80% and one sided significance level of 0,025. They
needed to include way less patients because most patients in the vancomycin
control groups showed recurrent CDI. Therefor the data and safety monitoring
board recommended closure of the trail. The method stayed the same during the
trial.
Study hypothesis
The
hypothesis stated in the article was a cure rate of 90% in the donor-feces
infusion group and a cure rate of 60% in both control groups.
Statistical analysis
Differences
in cure rates between the study groups were determined with Fisher's exact
probability test. The statistical significance of a change in microbiota abundance
was calculated with the use of a paired-samples Student t-test. Besides they
used a Wilcoxon signed-rank test.
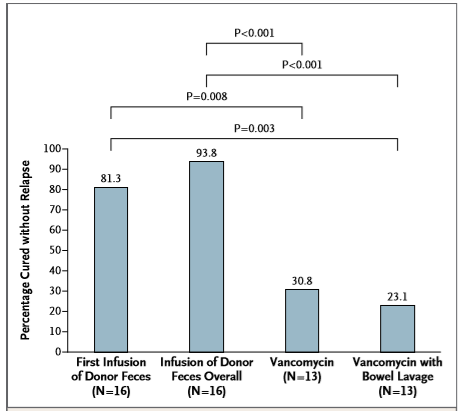
Conclusion of the article
The
conclusion of the article was: 'The infusion of donor feces was significantly
more effective for the treatment of recurrent C. difficile infection than the
use of vancomycin.' This is in line with the results. In the FMT group 81% was
cured after one infusion and 94% overall compared to a cure rate of 31% in the
vancomycin without bowel lavage group and 23% in the vancomycin group. Donor-feces
infusion was compared to both vancomycin groups statistically better.
(P<0.01 after first infusion, P<0.001 for overall cure rates). See figure
below.
Conflicts of interest
There are
no conflicts of interest. This study was supported by The Netherlands
Organization for Health Research and Development and performed in the Amsterdam
Medical Center.
My conclusion
Because of
the missing information about the treatment allocation concealment the
differences between the intervention and control group can be bigger than in reality.
This is an important fact that needs to be taken in account when reading this
article. Moreover, the number of participants in this study is very small. But
the recruitment of participants needed to be canceled because of ethical
reasons. All this taken in account I still think this is a good study to see
that the FMT indeed is a better therapy than vancomycin.
Written by Jodie Dekker
Posted on 14 oct 2018
Article of pictures and critical appraisal:
[1] Kelly, C. LaMont J. Clostridium difficile - More difficult than ever. In: The New England Journal of Medicine. N Engl J Med 2008; 359:1932-1940

